Polyvinyl alcohol: Properties, Production process and Uses
PVA is a water-soluble thermoplastic polymer prepared by partial or complete hydrolysis of PVAc with methanol or water. The chemical structure is:
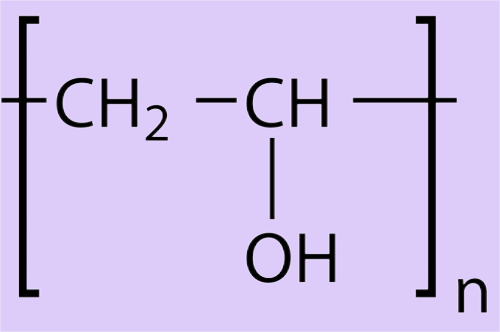
Properties of polyvinyl alcohol
PVA is an odorless white flake, flocculent or powdery solid. It is soluble in water, slightly soluble in dimethyl sulfoxide, but insoluble in gasoline, kerosene, vegetable oil, benzene, toluene, dichloroethane, carbon tetrachloride, acetone, ethyl acetate, methanol, ethylene glycol and so on.
The degree of polymerization of PVA is divided into ultra-high degree of polymerization (molecular weight of 250,000–300,000), high degree of polymerization (molecular weight of 170,000–220,000), medium degree of polymerization (molecular weight of 120,000–150,000) and low degree of polymerization (25,000–35,000). The degrees of alcoholysis are generally 78%, 88% and 98%. The degrees of alcoholysis for partial alcoholysis are usually 87–89%, and the degree of alcoholysis for complete alcoholysis is 98–100%. For product type of PVA, the thousand and hundred digits of the average degree of polymerization are usually put in the front, and the percentage of alcoholysis degree is put in the back. For example, PVA 17-88 means PVA with a polymerization degree of 1,700 and an alcoholysis degree of 88%. In general, as the degree of polymerization increases, the viscosity of the aqueous solution increases, and the strength and solvent resistance after film formation increase, but the solubility in water and the elongation after film formation decrease.
The relative density of PVA (25°C/4°C) is 1.27–1.31 (solid) and 1.02 (10% solution). It has a melting point of 230°C and a glass transition temperature of 75–85°C. It will change color when heated to above 100°C in the air. When heated to 160–170°C, dehydration and etherification occurs and the solubility is lost. PVA starts to decompose at 200°C.
High Purity 99% pva industrial grade polymer powder polyvinyl alcohol pva good price pva 1788 2488 powder
Process for manufacture of polyvinyl alcohol
PVA prepared by partial or complete hydrolysis of PVAc with methanol. In the presence of sodium hydroxide catalyst, three reactions in the methanol solution of PVAc occur as follows:

In the production process of PVA, there are two kinds of alkaline alcoholysis processes, wet process and dry process, which are often referred to as high alkali method and low alkali method. In wet alcoholysis process, the methanol solution of PVAc contains 1–2% of water, and the sodium hydroxide catalyst is prepared in the form of an aqueous solution. The amount of the alkali used is large and the reaction solution has higher alkali concentration. Therefore, it is also known as high alkali method. The advantage of high alkali method is that the speed of alcoholysis is faster and the production capacity of the equipment is higher. However, the disadvantage is that side reaction for generation of sodium acetate increases. The content of sodium acetate in PVA product is high, resulting in low purity of PVA and high ash content, which affects the quality of the product.
Dry alcoholysis means that the water content in the methanol solution of PVAc is less than 1%, and the alcoholysis is carried out almost in the absence of water. The amount of alkali used is small, and the consumption of sodium hydroxide is only 1/10 of that in the wet alcoholysis process. Moreover, the side reaction to sodium acetate is greatly decreased. Therefore, dry alcoholysis has become the main process for production of PVA. However, alcoholysis reaction is slower in the dry alcoholysis process.
In the dry alcoholysis process, the concentration of PVAc in the methanol solution is about 35%, and the reaction is carried out at a temperature of 40°C for 15–20 min.
Uses of polyvinyl alcohol
PVA is used in the manufacture of construction glue, polyvinyl acetal, vinylon, fabric treatment agent, dispersant, paper coating, adhesive and so on.
-
Premium Detergent Grade HPMC Hydroxypropyl Methylcellulose: Superior Thickening & StabilityNewsAug.31,2025
-
HEC 100000 Hydroxyethylcellulose for Paint | Superior ThickeningNewsAug.30,2025
-
Wall Putty Rdp Powder Packaging DesignNewsAug.29,2025
-
Introduction to Hpmc Hydroxypropyl Methyl CellulosNewsAug.29,2025
-
Hpmc Industri Grade IntegrationNewsAug.29,2025
-
How to Choose the Right Construction AdhesiveNewsAug.29,2025





